The UDI directive requires transmission into and management of UDI related data into GUDID (Global Unique Device Identification Data Base), the FDA's global data base.
GUDID serves the registration of medical devices and related manufacturers' data. Any device registered can be accessed by the general public, as well as everybody concerned professionally. As a future option, GUDID is to be linked to other data bases around the world, strongly promoting global recognition.
Based on universal standards for medical device identification, the data base comprises a key set of identifying elements for each possible UDI coding.
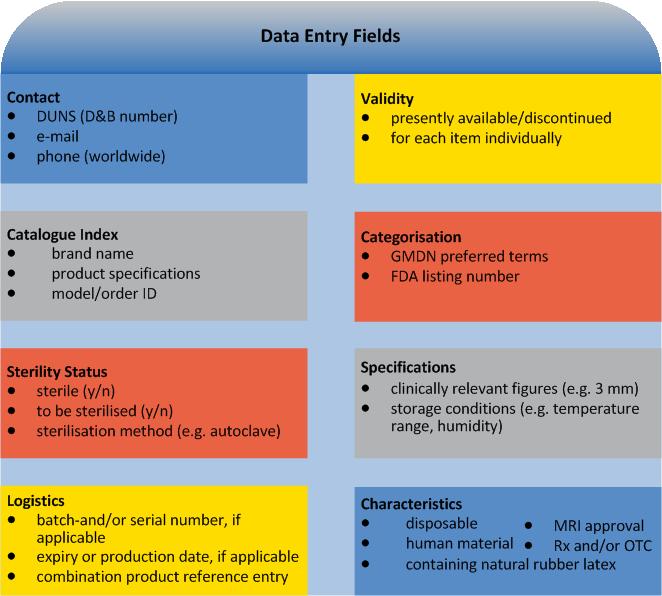
For each Device Identifier (DI) (no PIs): Trade mark proprietor, trade/brand name of the device. And furthermore:
applicable for new versions/models only: previous DI
type/model Number
applicable for directly marked devices only: DI other than indicated on type plate
dimensions of type/model
type of PI on type plate
FDA premarket submission and listing number(s)
Global Medical Device Nomenclature (GMDN) term
FDA product code (procode)
the number of individual devices in each package
commercial distribution status
higher levels of packaging
indication whether it is a kit, combination product or HCT/P
Furthermore UDI provides information on the individual device marking it:
as sterile or to be sterilised before use (nature and extent of sterilisation), advanced levels of packaging
as containing natural rubber latex
in respect of MRI compatibility (safe, conditional, unsafe)
as Rx and/or OTC
Methods of dato submission:
- Utilisation of the web tool
- Upload as HL7 XML file, utilising available tools
- Paid third party upload




