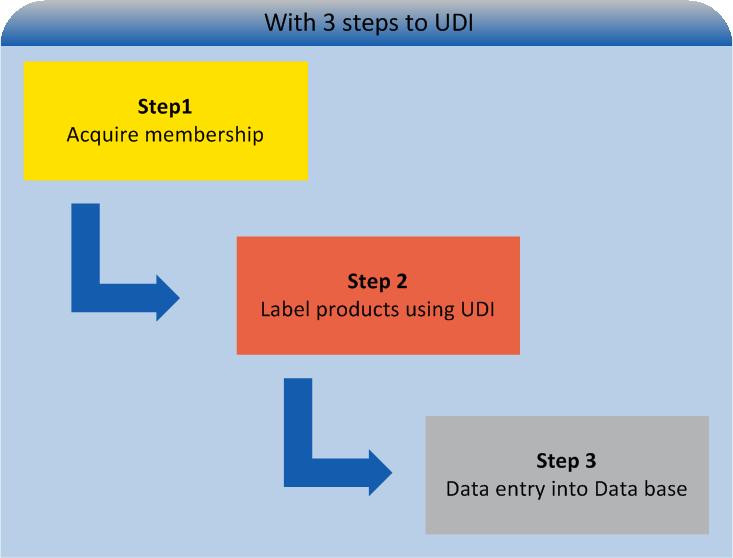
Currently more than 2,000 companies apply UDI-accredited HIBC codes. Among members, UDI can already be implemented quickly and without any difficulty (see: UDI for HIBC members). Manufactures, willing to utilise HIBC in the future, have to take three steps only. On the following pages you will find all relevant information, needed to label your products UDI-compliant.
UDI for HIBC members
To those, using HIBC codes already, UDI-compliant product labelling will be a simple task. Authorities in their implementation of UDI, refer to common practice. Referring to the good practice approach, there is no such thing as a new UDI code. First of all there is „ISO/IEC 15459 Unique Identification“, comprising registration rules for accreditation offices of singular company codes. Accordingly singularity of all UDI-DI is ensured by means of the preset sequence <accreditation office><labeller's code><product/device>.
Current HIBC members have to undergo an audit, following a quality inspection, ensuring UDI-DI and UDI-PI are applied correctly to the appropriate level of packaging.
Data transfer onto the central GUDID data base via the „HL7 SPL“-form is new, however. Doing so, all data entries, with the exception of the DUNS-Code or the GMDN classification code, which may be new to some manufacturers, rely on data already available. Nevertheless, these are open source codes; DUNS numbers are free of charge.
 HIBC-labelled products and devices are easily marked with the UDI-Code. UDI-compliant labels will automatically be clearly equipped with the HIBC badge showing, the manufacturer is well prepared for global developments, at a glance. The HIBC badge is proof for UDI compliance!
HIBC-labelled products and devices are easily marked with the UDI-Code. UDI-compliant labels will automatically be clearly equipped with the HIBC badge showing, the manufacturer is well prepared for global developments, at a glance. The HIBC badge is proof for UDI compliance!
HIBC – Healthcare Barcode
The HIBC Healthcare Barcode is a unique global product identifier, comprising extended product data in a concise data structure. Developed back in 1986 to ensure high-security track and trace, HIBC is recognised for one-of-a-kind security until present.
HIBC is a widely recommended global application within the ISO 22742 Standard for labelling product packaging. The standardised HIBC structure is compliant with all alphanumeric ISO-Symbologies, as well as with RFID (Radio Frequency Identification). Manufacturer- and product codes (REF), consisting of up to 18 alphanumeric digits, comprising entry fields for quantity, LOT, serial number, expiry and manufacturing dates, are supported. The extended form includes add-on information, e.g. integrated hyperlinks (URL).
REF in combination with a manufacturer ID, serve as unique global reference for scanning and entries into the UDI data base. HIBC contains all information in a transparent manner. Secondary encoding to identify manufacturer and item number is not necessary.
Benefits
HIBC is a unique product code, containing planned and actual data over the product's entire life cycle.
HIDC is fully UDI accredited by the FDA (Food and Drug Administration), as well as by the IMDRF (International Medical Device Regulatory Forum).
The REF number is directly included into the HIBC code.
HIBC innovatively utilises identical code symbols on packaging and device.
HIBC symbologies are adequate for labelling smallest devices.
HIBC is fully compliant with other coding systems.
The HIBC structure
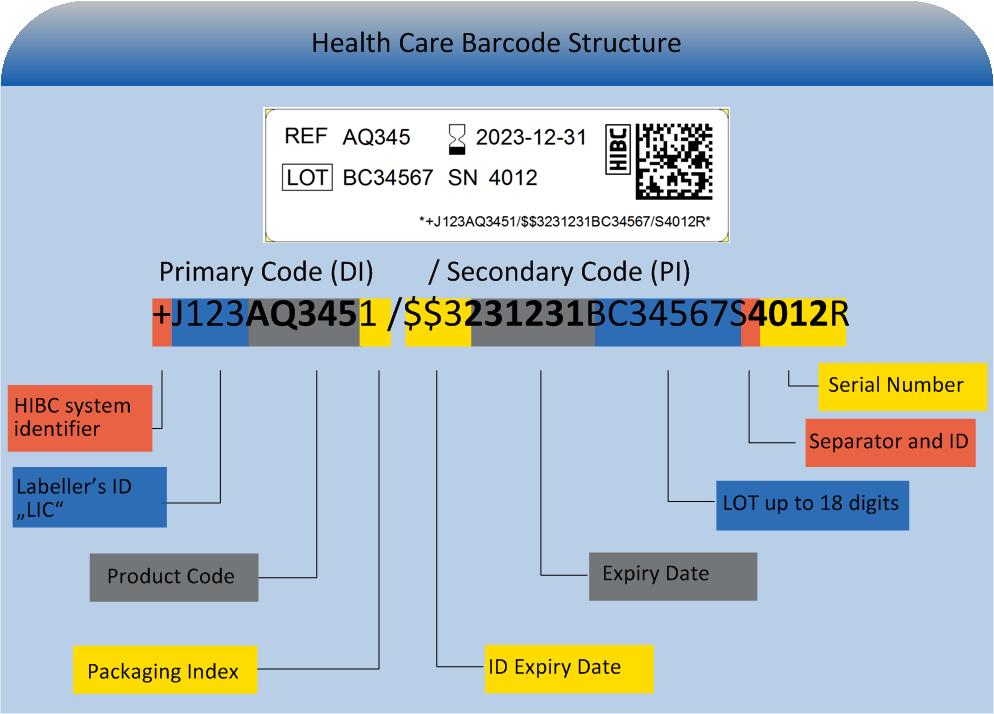
The HIBC code is composed of the primary code (UDI"DI"), containing the product reference, a "/" as delimiter symbol, the secondary code (UDI "PI") containing production data and a terminatory Modulo 43-compliant check character.
Primary code (UDI-DI)
The primary code (UDI"DI") builds a unique product reference incorporating these data fields:
- HIBC system identifier, all HIBC codes start "+"
- Labeller's ID, unique, clearly assigned, globally recognised (LIC = Labeller Identification Code)
- Product Code: 1-18 digits, alphanumeric, e.g. order number, lacking special characters (-,/)
- Packaging index: Referring to level of packaging. 0: single unit, 1: retail unit, 2-8: bulk package, 9: variable.
- Clearly assigned primary code for UDID data base entry
Secondary code (UDI-PI)
The secondary code (UDI-PI), containing production data is designed variably. It may contain the following entries:
- quantity: 2-5 digit number, containing dimension unit within master data
- expiry date in 7 possible computing formats
- LOT: 0-18 digits, alphanumeric, blank for no LOT
- serial number 1-18 digits, alphanumeric
- production date
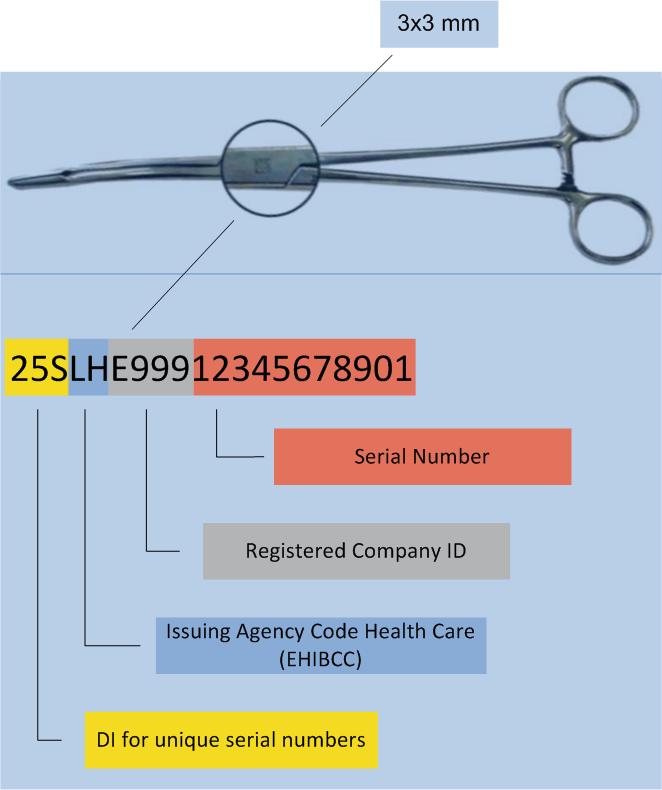
Direct labelling on smallest surfaces
Legal provisions require direct labelling of reprocessed medical devices (e.g. surgical instruments), as well as implants, requiring sterilisation.
To put small surface labelling to work, HIBC utilises the „Unique Identification Mark“ (UIM) data structure.